Researchers discover the common evolutionary history of gut microbes and their human hosts
The human gut microbiome includes thousands of different bacteria and archaea that vary greatly between populations and individuals. Researchers at the Max Planck Institute for Biology in Tübingen have now discovered the common evolutionary history of the gut microbiome and their human hosts: The human gut microbiota has evolved over hundreds of thousands of years in parallel with humans. In addition, some microbes have functional and genetic characteristics that make them dependent on the human gut environment. The research team is now presenting the results of a study conducted using data from 1,225 people from Africa, Asia and Europe.
© Source: MPI for Biology.
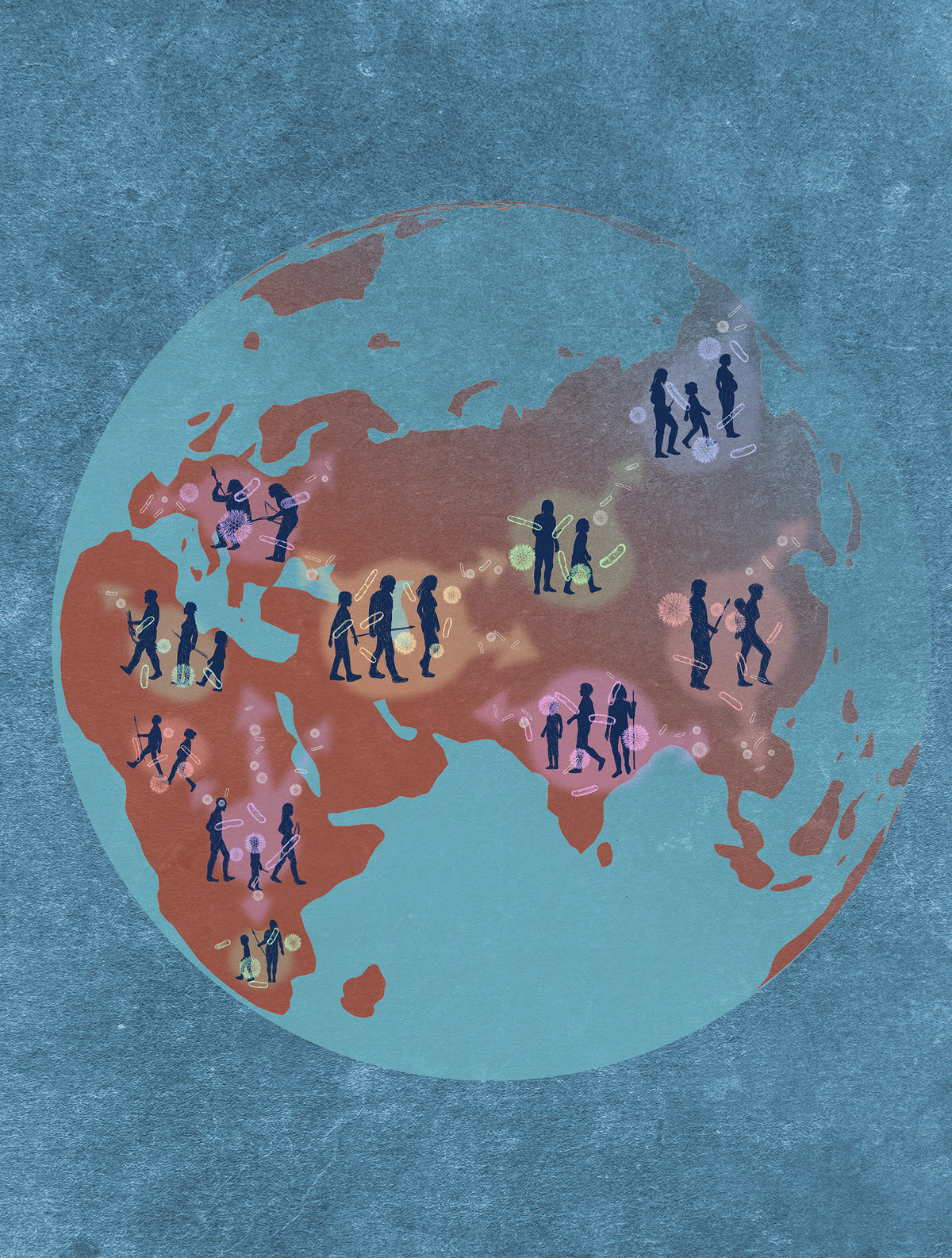
Gut microbes spread throughout the world with their human hosts. © Source: MPI for Biology.
Many microbial species are found in the human gut in diverse groups throughout the world. However, microbial strains within microbial species vary greatly between individuals and populations. Despite their importance to human health, little is known about the evolution of the different breeds. In view of the fact that most of these strains occur almost exclusively in the human intestine, the question of their origin arises.
The research team at the Max Planck Institute for Biology in Tübingen hypothesized that some species and strains that accompanied humans as they spread around the world and evolved in parallel in their gut. To do this, researchers from the Max Planck Institute for Biology, the Institute of Tropical Medicine and the CMFI Excellence Group at the University of Tübingen have systematically compared the evolutionary history of humans and gut microbes for the first time. Researchers created a family tree for 1,225 people tested on humans and 59 types of microbes from their gut. Using statistical tests, they analyzed the concordance of these family trees.
More than 60 percent of the species studied showed a relative history parallel to their human host. This indicates that these microbes evolved in the human gut over hundreds of thousands of years as humans spread across continents from Africa. “We never knew that the microbes in our gut followed our evolutionary history so closely,” said Ruth Lee, head of microbiome research at the Max Planck Institute for Biology in Tübingen, where the study was conducted, and CMFI’s deputy spokesperson.
The gut microbiome is human-dependent
“It is also worth noting that those lineages that closely followed our evolutionary history are now more dependent on the gut environment,” Lee adds. In fact, some microbial strains that have evolved with humans are highly dependent on the human gut environment: they have smaller genomes and are more sensitive to changes in oxygen levels and temperature — traits that make it difficult to survive outside the human body.
In contrast, microorganisms that showed a weaker connection to human history showed more characteristics of free-living bacteria. “Some gut microbes behave as if they were part of the human genome,” explains Taichi Suzuki, first author of the study with colleague Liam FitzStevens. Suzuki adds: “These microbes are sort of somewhere in the spectrum from ‘free-living’ to dependence on the environment of the human body. We were able to show that some human gut bacteria have progressed along this spectrum towards irreversible dependence than previously thought. Ley further adds: “These findings fundamentally change our view of the human gut microbiome.”
Microbiome therapies adapt to populations
To obtain data from a diverse sample of different populations around the world, the research team analyzed the gut microbes and genomes of 1,225 people in Europe, Asia and Africa. Stool and saliva samples were collected with the help of researchers from the Institute of Tropical Medicine at the University of Tübingen and their partner organizations in Vietnam and Gabon. In addition, researchers around the world supported the study with comparable data sets from participants from Cameroon, South Korea, and the United Kingdom.
The study findings contribute to a better understanding of the microbes that have long been part of certain populations. With this knowledge, microbiome disease therapies can be better adapted and refined for the local population involved.

“Award-winning music trailblazer. Gamer. Lifelong alcohol enthusiast. Thinker. Passionate analyst.”





More Stories
Wimbledon 2025 goal – Boris Becker is no longer insolvent – Culture and Entertainment
Former tennis star: Wimbledon 2025 goal – Boris Becker is no longer insolvent – Entertainment
Former tennis star: Wimbledon 2025 goal – Boris Becker is no longer insolvent – Entertainment