Researchers have made a discovery that may facilitate the storage of excess energy. Electrolytes are central here.
The basics in brief
- Energy storage is a big topic.
- EPFL researchers have now cleared an obstacle along the way.
- Focus here: Electrolysis reactions.
when using from renewable energies Storage is basic: backlog energySurpluses must be saved and used again Convert it into energy can become. Researchers at EPFL have made a discovery that could make this easier. One method currently being explored by many researchers is to store energy In gaseous form in electrolysis cells.
These run on electricity, which leads to an electrolysis reaction in which water molecules split into hydrogen and oxygen. Electricity can then be generated by reversing the reaction and recombining hydrogen and oxygen water recover.
The electrolysis reactions of water were examined
Catalysts play a critical role in this reaction. They are used to speed up electrocatalytic reactions without consuming them. The catalysts used in the electrolysis of water are metal oxides, some of which tend to work better than others, but the exact reason for this was not previously known.
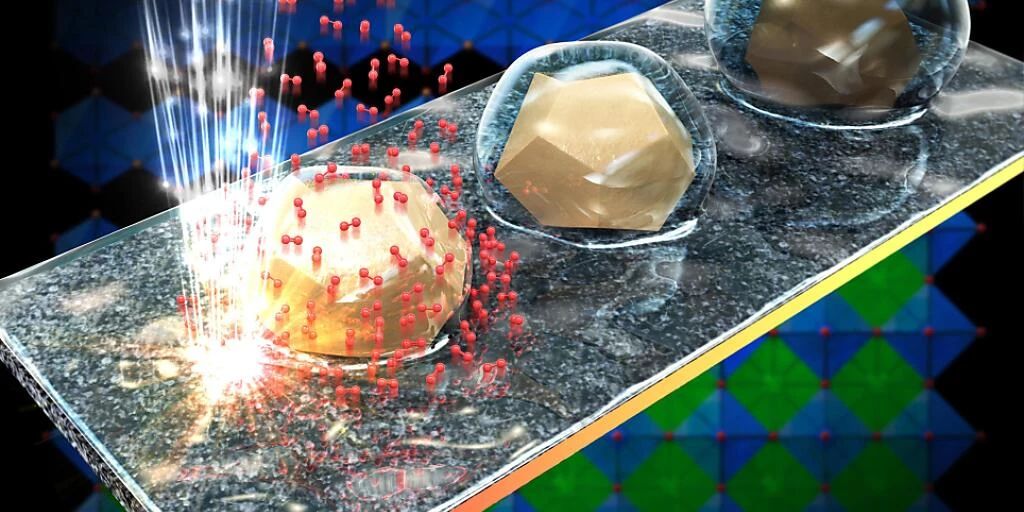
To find out, Vasiliki Tellili and her graduate student Tzu Hsin Shin observed the electrolysis reactions of water under an electron microscope. They studied how the catalyst behaves during the process by creating nanophotographs.
Redistribution of surface atoms
They used a perovskite-type oxide catalyst called BSCF. “It’s a fascinating catalyst with extraordinary water-splitting properties,” says Tellie, associate professor and head of the EPFL Laboratory for In Situ Characterization of Electron Nanomaterials. “Most of the catalysts currently in use, for example b. iridium and ruthenium, although effective, are very expensive and the supply is limited. So alternatives must be found.”
While observing and documenting the behavior of the catalyst, the researchers found that the surface atoms of the particles were redistributed during the reaction and the surface properties changed. As a result, molecules interact differently with their environment at different stages of the electrolysis cycle. Some steps have a water repellent coating, while others do water attracts.
This was suspected but not previously observed at the nano level and in real time. According to Tileli, the material’s ability to switch between a hydrophobic and a hydrophilic state is very valuable to engineers and can be used in a wide range of applications: for example in sensors, water purification systems and self-cleaning surfaces.
the study It was published in the journal Nature Catalyses.

“Prone to fits of apathy. Zombie ninja. Entrepreneur. Organizer. Evil travel aficionado. Coffee practitioner. Beer lover.”





More Stories
Millions of Windows users benefit: New features are ready for abandoned systems
NIKKOR Z 28-400mm f/4-8 VR – a lightweight superzoom for Nikon Z cameras
Smart Pro Camera: Samsung Galaxy S24 Ultra